- KYPROLIS® (carfilzomib) is indicated in combination with dexamethasone, or with lenalidomide plus dexamethasone, or with daratumumab plus dexamethasone, ... Read More Close
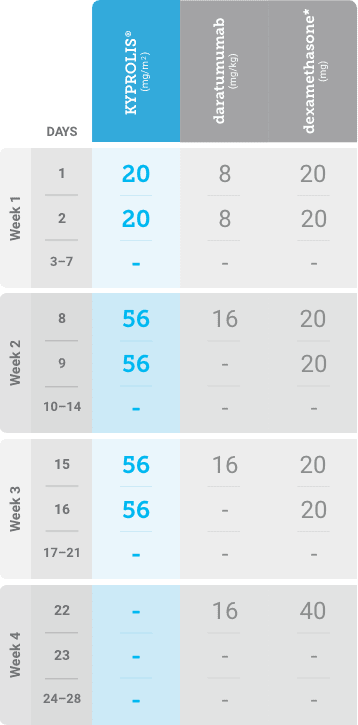
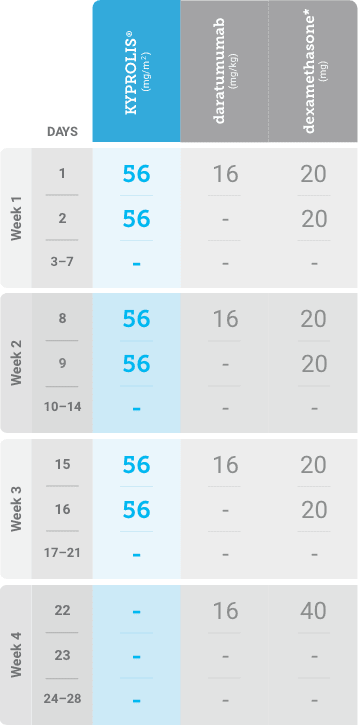
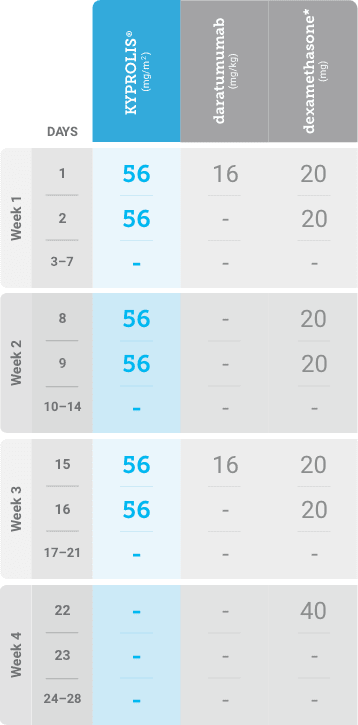
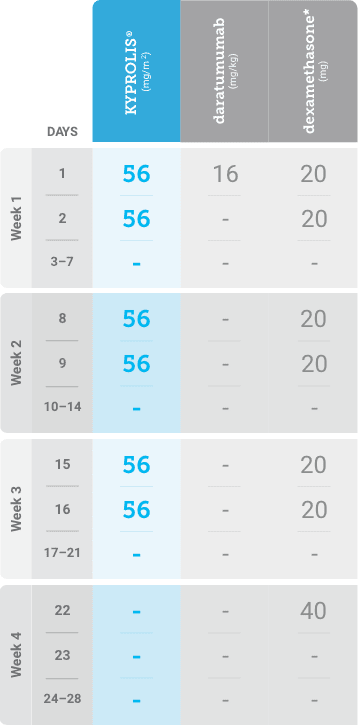
Meet your patients’ needs with flexible dosing options, including an option with subcutaneous daratumumab1,*
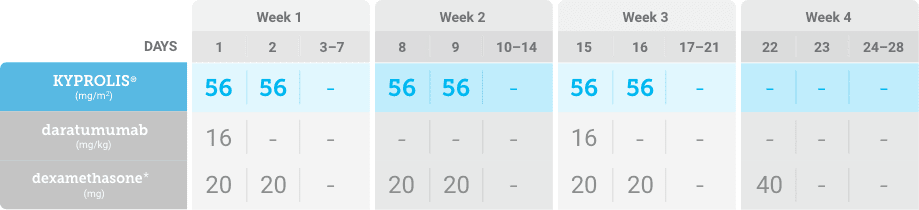
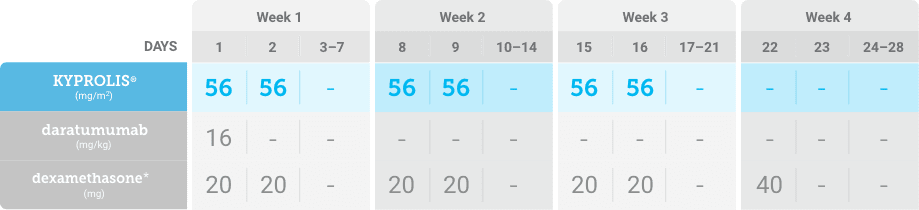
KYPROLIS® is approved in combination with both daratumumab for intravenous infusion and daratumumab and hyaluronidase-fihj for subcutaneous injection.1
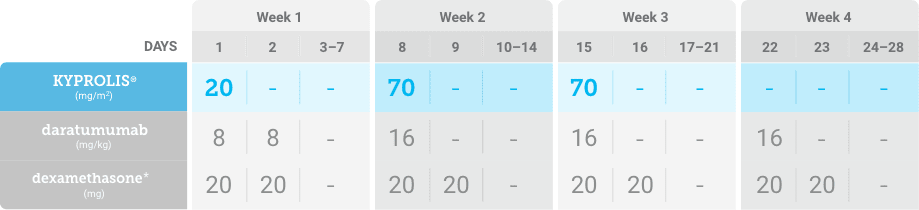
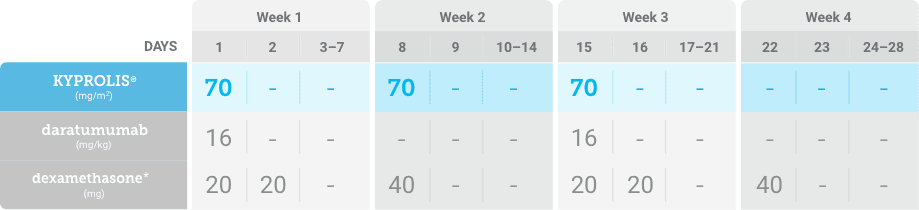
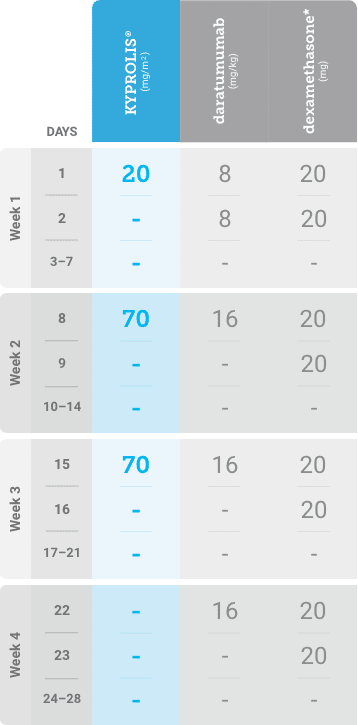
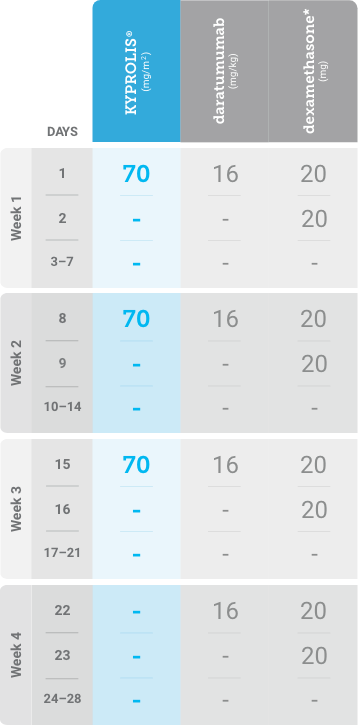
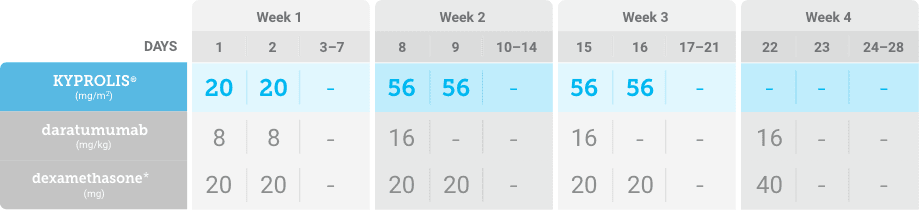
Phase 1b, open-label, multicohort study that evaluated the combination of once-weekly KYPROLIS® 70 mg/m2 with intravenous daratumumab plus dexamethasone (DKd) in a cohort of 85 patients with RRMM who had received 1 to 3 prior lines of therapy. KYPROLIS® was administered weekly on days 1, 8, and 15 of each 28-day cycle at a dose of 70 mg/m2 with a priming dose of 20 mg/m2 on day 1 of cycle 1. Safety and tolerability of DKd were evaluated as primary endpoints. Overall response rate and overall survival were evaluated as secondary endpoints. Results from the EQUULEUS study explored DKd regimen safety and efficacy that informed the phase 3 CANDOR study and provided the rationale for the once-weekly dosing of DKd for patients with RRMM who had received at least one prior line of therapy.2
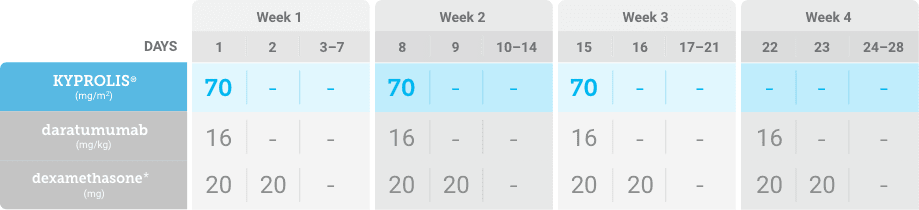
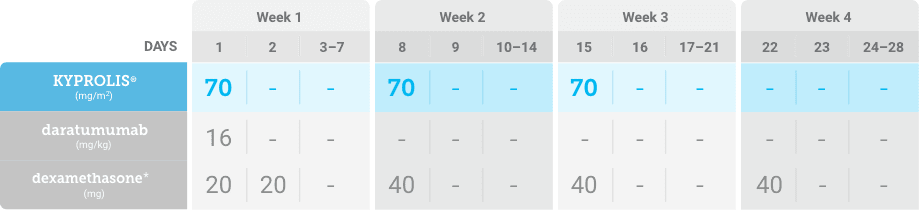
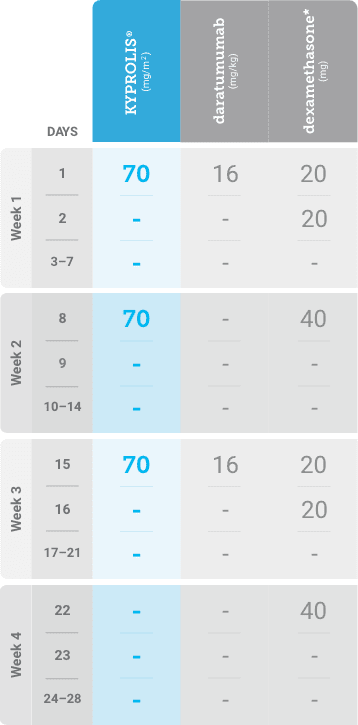
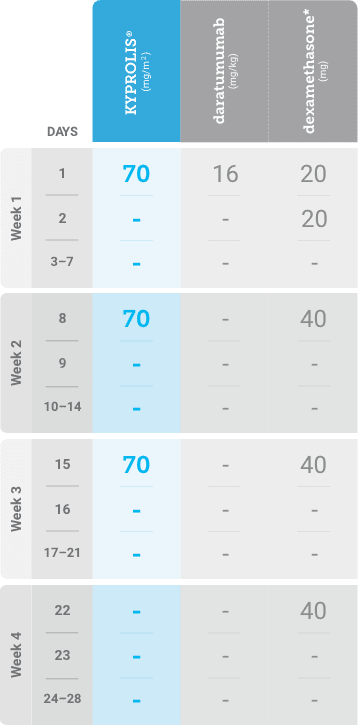
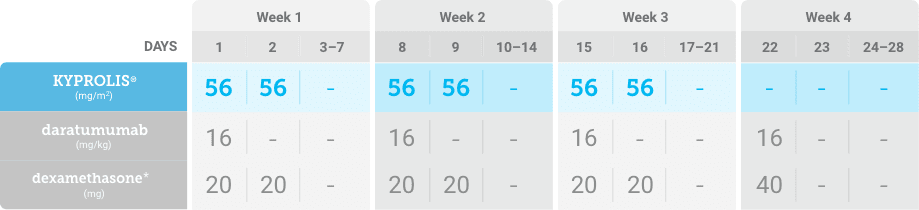
Phase 2, open-label, multicenter trial that evaluated the combination of KYPROLIS® 70 mg/m2 once weekly with subcutaneous daratumumab and hyaluronidase-fihj plus dexamethasone (DKd) in a cohort of 66 patients with RRMM who had received only one prior line of therapy.3
DKd, carfilzomib + daratumumab + dexamethasone; RRMM, relapsed or refractory multiple myeloma.
Please see accompanying full Prescribing Information.
Please see accompanying full Prescribing Information.
References: 1. KYPROLIS® (carfilzomib) prescribing information, Onyx Pharmaceuticals Inc., an Amgen Inc. subsidiary. 2. Chari A, Martinez-Lopez J, Mateos MV, et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood. 2019;134:421-431. 3. Chari A, Rodriguez-Otero P, McCarthy H, et al. Subcutaneous daratumumab plus standard treatment regimens in patients with multiple myeloma across lines of therapy (PLEIADES): an open-label Phase II study. Br J Haematol. 2021;192:869-878.