- KYPROLIS® (carfilzomib) is indicated in combination with dexamethasone, or with lenalidomide plus dexamethasone, or with daratumumab plus dexamethasone, ... Read More Close
Target the 27 mg/m2 dose for the best chance of achieving outcomes observed in the clinical trial
Infusion time
10 minutes
KYPROLIS® priming dose
20 mg/m2 on Days 1 and 2 of Cycle 1 to evaluate tolerability
Target KYPROLIS® therapeutic dose
27 mg/m2 starting Day 8 of Cycle 1
Treatment schedule
KRd, carfilzomib + lenalidomide + dexamethasone; Rd, lenalidomide + dexamethasone.
In Cycle 1, the 20 mg/m2 dose is used for Days 1 and 2 to evaluate tolerability. Target the 27 mg/m2 dose of KYPROLIS® starting on day 8 of Cycle 1 if the priming dose is tolerated on Days 1 and 2 of Cycle 1.1
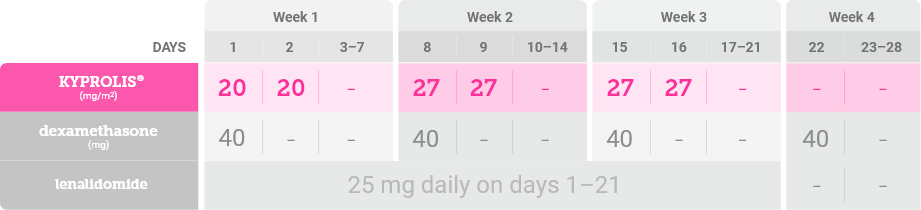
In Cycles 2–12 and beyond, the targeted label dose of KYPROLIS® is 27 mg/m2 as tolerated1,*
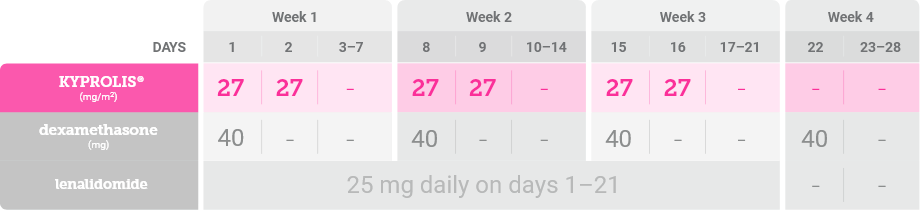
*Until disease progression or unacceptable toxicity.
From Cycles 13–18, KYPROLIS® is dosed on consecutive days every other week1,*
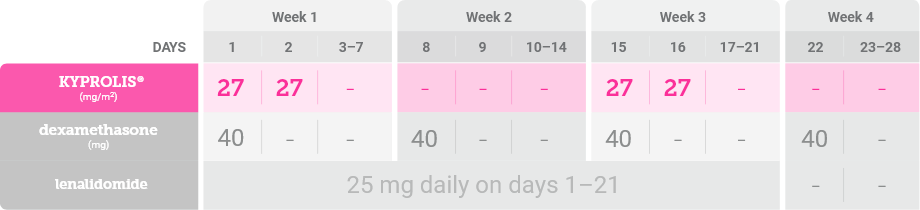
*Until disease progression or unacceptable toxicity. Discontinue KYPROLIS® after Cycle 18.1
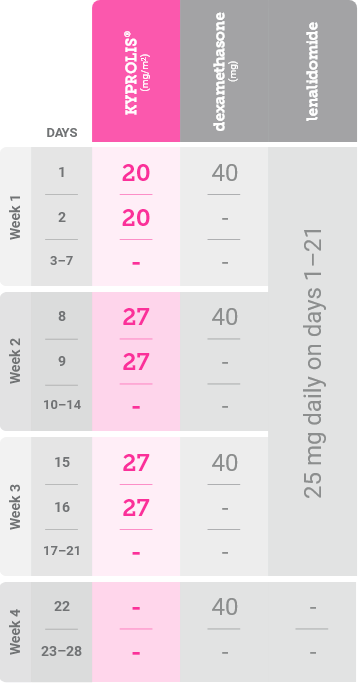
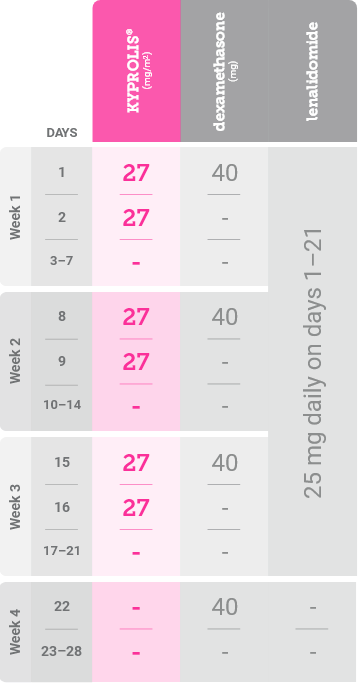
*Until disease progression or unacceptable toxicity.
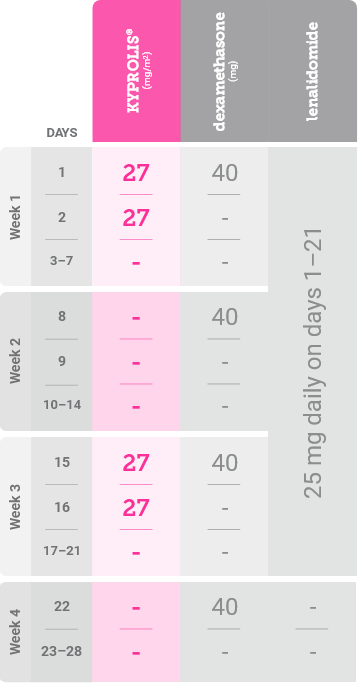
*Until disease progression or unacceptable toxicity. Discontinue KYPROLIS® after Cycle 18.1
Please see accompanying full Prescribing Information.
Please see accompanying full Prescribing Information.
Reference: 1. KYPROLIS® (carfilzomib) prescribing information, Onyx Pharmaceuticals Inc., an Amgen Inc. subsidiary.